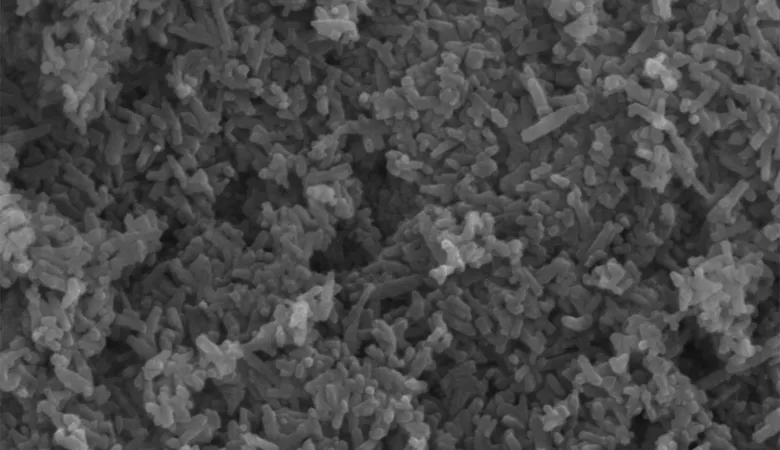
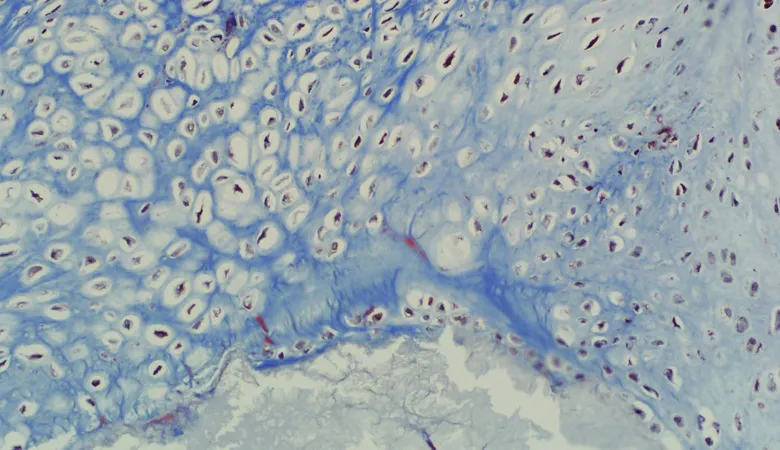
OssDsign Catalyst is now indicated for interbody fusion
– The first synthetic bone graft to be cleared to market for interbody use based on bone graft data alone, allowing on-label use in any interbody cage cleared for use with synthetic bone grafts.